Guide to Irradiation and Sterilization Validation of Single-Use Bioprocess Systems - BioProcess InternationalBioProcess International
+A1-2013.jpg)
ANSI/AAMI/ISO 11137-1:2006 (R2010)/A1:2013 - Amendment 1 - Sterilization of health care products - Radiation - Part 1: Requirements for development, validation, and routine control of a sterilization process for medical devices

ISO 11137-1:2006, Sterilization of health care products - Radiation - Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices: ISO/TC 198: Amazon.com: Books

ISO 11137 performance qualification-a relatively narrow dose mapping... | Download Scientific Diagram

ISO 11137 Part 1 - Radiation - BRITISH STANDARD BS EN ISO 11137-1: Sterilization of health care - Studocu
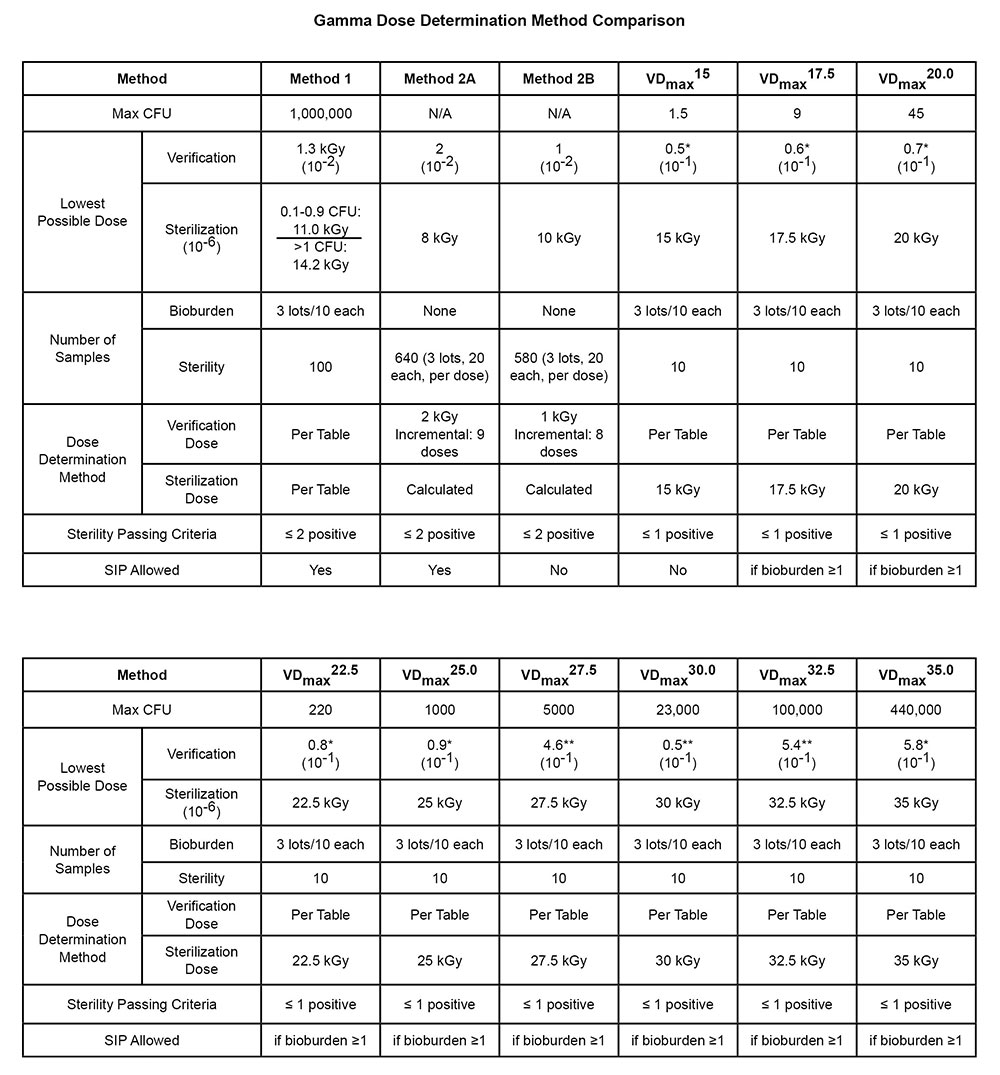
Comparison of AAMI Methods for Setting of Minimum Sterilization Dose with Irradiation | TechTip | STERIS AST
UNE EN ISO 11137-1:2015/A2:2020 Sterilization of health care products - Radiation - Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices - Amendment 2: Revision to 4.3.4 and 11.2 (ISO 11137 ...
40[85:AOOTRR]2.0.CO;2/asset/eefab0e4-c696-4c3f-b163-b6569c5dba92/assets/graphic/i0899-8205-40-1-85-t01.gif)
An Overview of the Revised Radiation Sterilization Standards | Biomedical Instrumentation & Technology

ISO 11137-1:2006 - Sterilization of health care products - Radiation - Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices